현재 사용 중인 언어로는 이 페이지를 사용할 수 없습니다. Google Translate을 사용하여
자동 번역된 페이지
를 볼 수 있습니다. Renishaw에게는 이 서비스를 제공할 책임이 없으며 번역 결과를 저희가 확인하지도 않았습니다.
추가로 도움이 필요하시면
저희에게 연락해 주십시오.
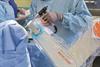
Renishaw neuromate® frameless Gen II cleared by the FDA
7 May 2014
Renishaw is pleased to announce that the U.S. Food and Drug administration (FDA) has issued clearance to market the neuromate® frameless Gen II stereotactic robotic system.
Dr. Abed Hammoud, CEO of Renishaw Mayfield SA said, “We are excited to launch our newest generation neuromate® robotic system in the US, which is the largest global market for medical devices. Our clinical solutions and technical support teams are looking forward to working with some of the leading centres in the US and establishing robotics in the Neuro operating room.”
For further information on Renishaw neurological products, visit www.renishaw.com/neurological.
Downloads
All images and text copyright Renishaw
Register for news updates
Register for regular news updates from Renishaw